Azure DevOps & App Quality Engineer - Medical Device
Role details
Job location
Tech stack
Job description
Location: Hybrid / Remote (UK based, with occasional visits to Nottingham and Basingstoke offices), You'll be the guardian of our cloud platform and mobile application, ensuring that every deployment, every update, and every release meets the exacting standards of medical device regulations while maintaining the reliability our customers depend on.
This is a critical role. Our customers trust our technology with their wellbeing. Your work ensures that trust is never broken.
What you'll do
Own the infrastructure
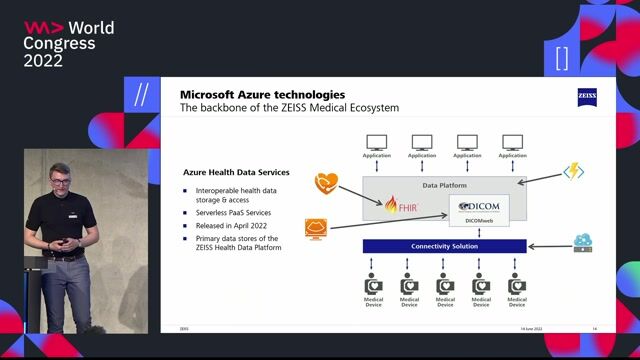
- Manage our Azure ecosystem and build CI/CD pipelines that automate our software development lifecycle.
- Monitor system health, performance, and security-because downtime isn't an option.
Guarantee quality and compliance
- Lead validation and verification (V&V) activities for every release-producing audit-ready evidence.
- Design and execute comprehensive testing strategies and ensure full traceability.
- Maintain compliance with ISO 13485, IEC 62304, ISO 27001, and EU MDR (2017/745).
Protect what matters
- Implement cybersecurity controls and security best practices (GDPR, HIPAA).
- Support investigations, incident response, and maintain documentation for regulatory audits.
Bridge the teams
- Collaborate with external development partners and internal QA/RA teams.
- Act as the technical liaison ensuring smooth software delivery across all stakeholders.
Requirements
Do you have experience in iOS?, * 5+ years in Azure cloud infrastructure and DevOps roles.
- Proven experience in software testing within regulated or safety-critical environments.
- Hands-on knowledge of Azure DevOps pipelines, App Services, and version control systems.
- Understanding of medical device regulations (ISO 13485, IEC 62304, EU MDR).
Bonus points for:
- Experience with Class II/III medical device software or SaMD.
- Mobile app testing (iOS/Android).
- Programming skills (JavaScript/Node.js) and containerisation knowledge (Docker, Kubernetes).
Benefits & conditions
Make a tangible difference. Every day, you'll know that your work directly impacts the lives of people who've been searching for hope. You'll hear their stories. You'll understand the difference you're making.
Be part of something innovative. We're not following the market-we're creating it. Our technology represents a paradigm shift in how Tourette's syndrome is managed.
Work with purpose and autonomy. We're a quality-driven team that trusts our engineers to own their domains. You'll have the responsibility and the freedom to make this platform exceptional.
Grow your expertise. Continuous professional development in medical device compliance, cybersecurity, and Azure DevOps technologies.
Competitive package. Salary and benefits that reflect the critical nature of this role.
Ready to make history?
If you're a DevOps and quality engineer who wants their work to mean something and who wants to be part of a team that's genuinely changing lives, we would love to hear from you.
CV not up-to-date, or need more information before applying? Send our careers team an email on careers@neupulse.co.uk to arrange a call.
Neupulse is an equal opportunity employer. We celebrate diversity and are committed to creating an inclusive environment for all employees.